Mabqi’s technology is based on proprietary fully human and highly developable synthetic antibody suite of libraries combined to flexible and robust dual display technologies, especially fitted for challenging targets.
In vitro antibody libraries offer several advantages over traditional animal immunization strategies for the discovery and development of therapeutic antibodies. These libraries are key for generating diverse and high-quality antibodies, featuring the following benefits:
In vitro antibody libraries can achieve immense diversity, with sizes reaching up to 1011 independent clones. This significantly enhances the likelihood of identifying antibodies with high affinity and specificity for a wide range of antigens. Moreover, they enable the selection of antibodies against virtually any antigens including challenging targets such as GPCRs and ion channels.
Combinatorial antibody libraries can directly generate fully human antibodies, bypassing the immunogenicity concerns often associated with animal-derived antibodies
In vitro selection allows for screening antibodies with desired specific characteristics such as stability, species cross-reactivity, and high binding affinity, all within the selection process itself.
Antibodies produced in vitro can easily be converted into various formats such as: standard IgG, engineered IgGs (Fc-enchanced/Fc-silenced), Fab, single-chain variable fragments (scFv), ADC, bsAb). This versatility allows customization for specific therapeutic or diagnostic applications.
The discovery of an antibody fragment that could retain its functionality inside cells and be produced stably in very high yields by Pierre Martineau (co-founder of Mabqi) and Sir Greg Winter (Nobel Prize in 2018) in Cambridge in 1998 opened up the doors to the generation of antibody fragment libraries and corresponding full antibodies with high developability properties.
Over the years, these so called “naive” or “single pot” antibody phage libraries derived from this unique clone have been patented and used successfully to derive highly specific and stable therapeutic antibodies to a wide range of different targets (proteins, protein complexes, peptides, GPCR and ion channel).
Mabqi offers a range of human antibody libraries that integrate data from scaffold, bioinformatics and structure modelling to optimize stability, paratope diversity, and affinity. Rational structure-based design and best-in-class precision technology have been used for accurate synthesis to maximize functionality and developability.
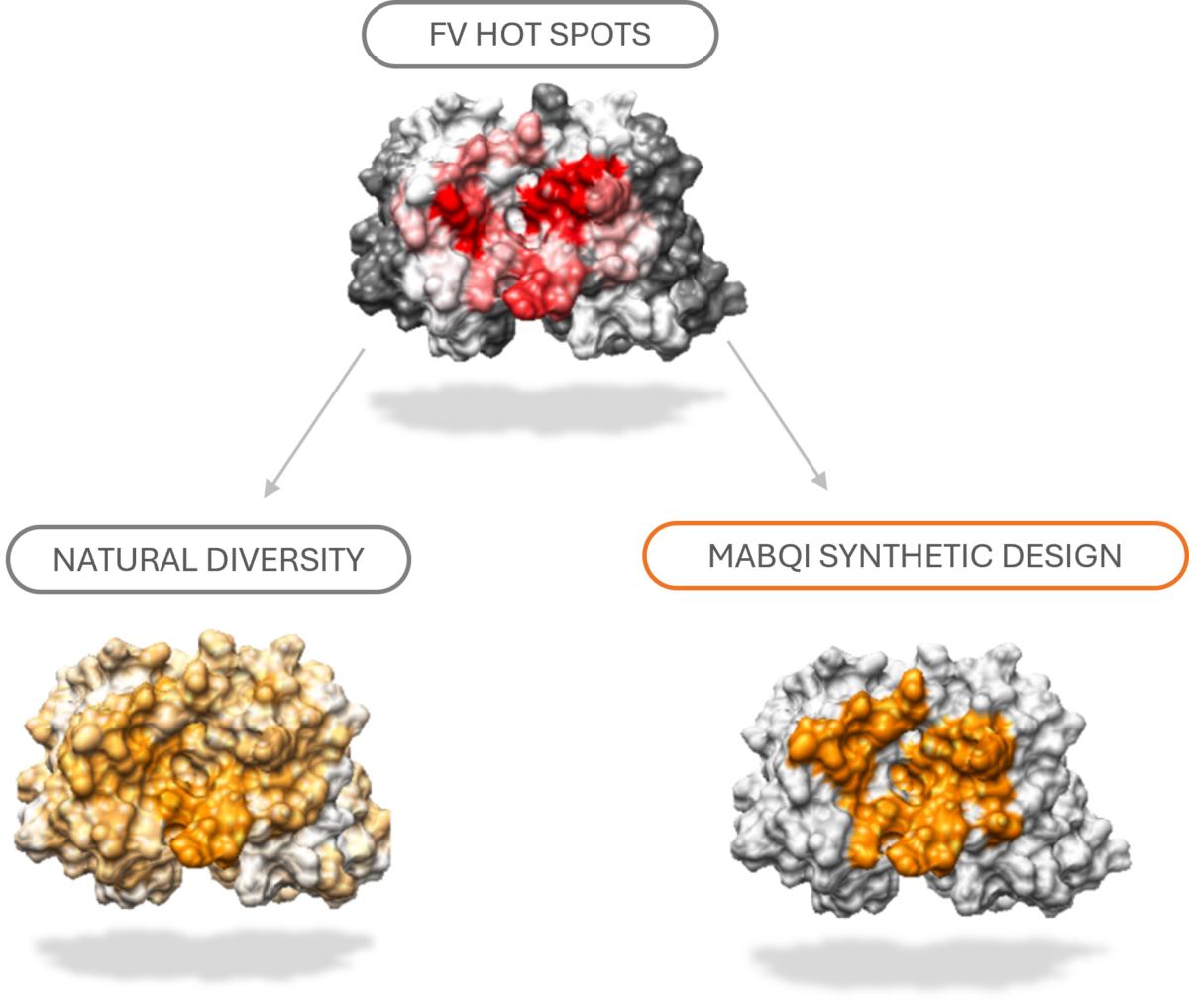
Our libraries are based on a single optimized scaffold reaching remarkably high levels of expression and stability.
Furthermore, the library true diversity (functional diversity >1010 with minimal risk for immunogenicity) has been carefully designed to retain the scaffold remarkable properties resulting in collections of selected antibodies with homogeneous high expression and stability.
Through extensive in silico analysis of hundreds of antibody-antigens crystal structures and model structures integrating the hyper-stable scaffold, key positions and residues were identified to optimize diversity for stability, affinity, and epitope coverage.
The human scaffold CDR residue usage and lengths variations are consistent with human antibodies.
Mabqi has developed a proprietary suite of synthetic 100% human antibody libraries:
The core strengths of our libraries encompass:
State-of-the-art in vitro display technologies, such as phage display and yeast display, have revolutionized the generation of antibodies, enabling the generation of high-quality antibodies virtually against any target of any type (peptides, proteins, whole cells).
These technologies facilitate human antibody generation and offer unique advantages over the traditional hybridoma approach:
Furthermore, the original naive antibody library can already be in human format (no more humanization needed) and selections can be designed to obtain species cross-reactive antibodies (eg. mouse-human-NHP) allowing better assessment of therapeutic efficacy and toxicity in animal models.
Mabqi has extensive experience in in vitro phage and yeast display technologies combined with highly optimized proprietary humanized synthetic antibody libraries from which antibody leads have been obtained for challenging targets: GPCR, ion channel, peptides, pMHC, …
Mabqi leverage its LiteMab© Antibody Discovery Studio with artificial intelligence and ML powerful cutting-edge tools for:
These AI-driven approaches significantly accelerate the antibody discovery process, improve the quality of candidates, and enhance the overall efficiency of therapeutic antibody development.