Mabqi leverages on its extensive antibody discovery expertise and cutting-edge platform to propose a pipeline of drug candidates for high, unmet clinical needs.
We focus our discovery engine on finding antibodies with outstanding characteristics including superior developability profiles, pH sensitivity, and/or binding to exclusive targets such as GPCR or ion channels.
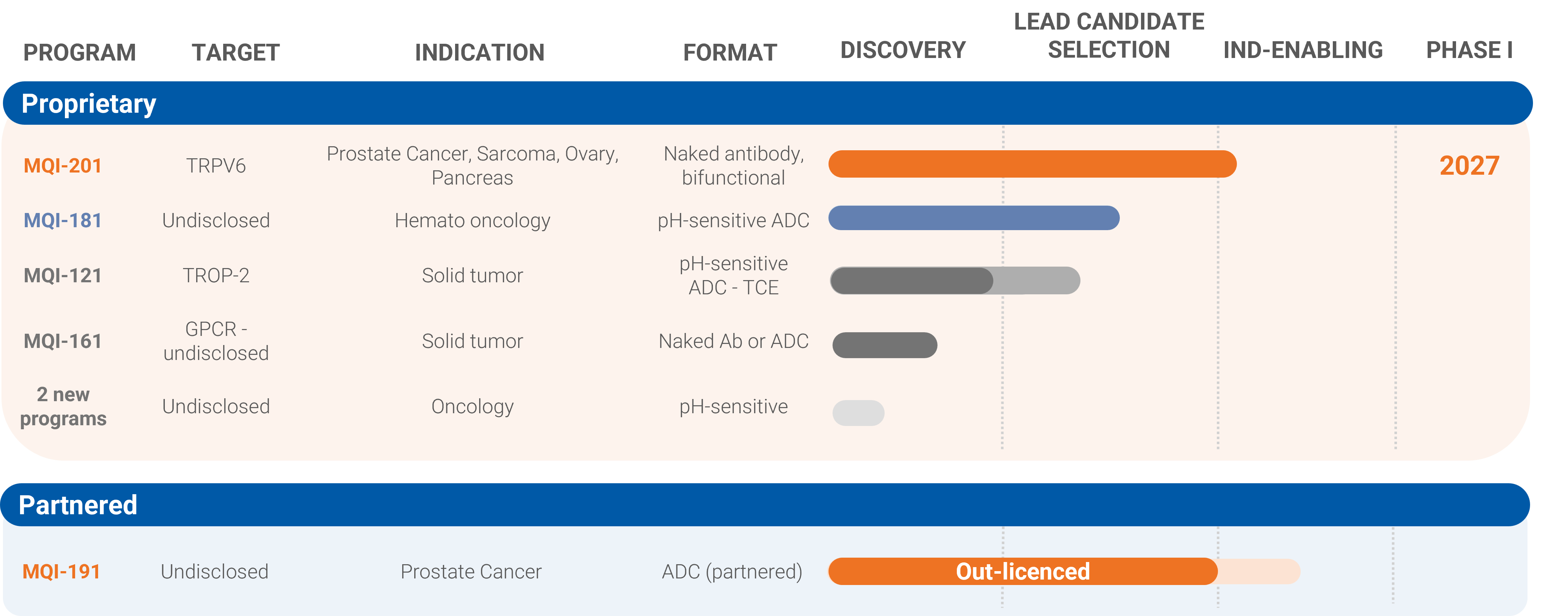
Mabqi, in collaboration with SATT Nord has discovered and characterized a first-in-class human anti-TRPV6 antibody with unique antitumoral properties and high clinical potential.
Comprehensive in vitro and in vivo preclinical studies have demonstrated the safety, efficacy, and clinical relevance of our lead antibody, positioning it as a promising new candidate for the treatment of metastatic prostate cancer, sarcoma and pancreas cancer.
This pioneering anti-TRPV6 antibody drug candidate is well-positioned for rapid clinical advancement.
Latest in vitro and in vivo results were presented at the American Association for Cancer Research (AACR) 2025. First-in-class anti-TRPV6 antibody as a new therapeutic agent in cancer. Cancer Res (2025) 85 (8_Supplement_1): 4770.
In 2025, Mabqi secures non-dilutive funding of €5 million as part of the France 2030 program to advance this lead antibody candidate into clinical development. More information in the press release.
A novel, tumor-specific immunotherapy designed for hematology-oncology indications, with the potential to evolve into a next-generation antibody-drug conjugate (ADC) for lymphoma and other blood cancers.
Latest preclinical in vivo results were presented at the American Society of Hematology (ASH) Annual Meeting in 2025.
Additional in vivo data are available, and the program is currently open for partnering opportunities in both preclinical and clinical development stages.
The ADC drug candidate was developed in partnership with an expert in ADC technologies. It has shown an excellent safety profile and robust antitumor activity, including complete tumor regression across various solid tumor models.
The drug candidate is progressing toward clinical evaluation in prostate cancer.
In 2026, the company successfully out-licensed the drug candidate. Access the full press release here.
The company has established a Medical Advisory Board to enhance its ability to identify and select innovative targets. This board comprises international experts in clinical medicine, pharmacology, and pharmaceutical development. The primary purpose of this advisory board is to provide strategic guidance on future product positioning.
Our oncology programs, focused on our lead candidates, are open for collaboration through partnering or out-licensing agreements.
We are committed to pushing the boundaries of immunotherapy by combining state-of-the-art technologies with groundbreaking concepts, aiming to deliver safer and more potent therapeutic solutions.
Furthermore, we are also open to explore partnership opportunities involving innovative targets and mechanisms of action that could be enhanced by our cutting-edge technology platform.
